All chemical changes are accompanied by the absorption or release of heat. The intimate connection between matter and energy has been a source of wonder and speculation from the most primitive times; it is no accident that fire was considered one of the four basic elements (along with earth, air, and water) as early as the fifth century BCE.
In this unit we will review some of the fundamental concepts of energy and heat and the relation between them. We will begin the study of thermodynamics, which treats the energetic aspects of change in general, and we will finally apply this specifically to chemical change. Our purpose will be to provide you with the tools to predict the energy changes associated with chemical processes. This will build the groundwork for a more ambitious goal: to predict the direction and extent of change itself.
One of the interesting things about thermodynamics is that although it deals with matter, it makes no assumptions about the microscopic nature of that matter. Thermodynamics deals with matter in a macroscopic sense; it would be valid even if the atomic theory of matter were wrong. This is an important quality, because it means that reasoning based on thermodynamics is unlikely to require alteration as new facts about atomic structure and atomic interactions come to light.


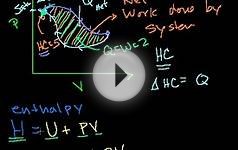






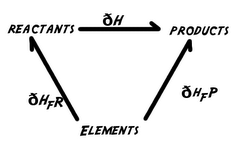 Hess' law is a relationship in physical chemistry named after Germain Hess, a Swiss-born Russian chemist and physician.
Hess' law is a relationship in physical chemistry named after Germain Hess, a Swiss-born Russian chemist and physician.